Topical eye drops are the therapeutic pillar for many ocular diseases, but traditional water-based formulations have significant limitations that may influence efficacy and safety.
EyeSol® is the worldwide first and only water-free technology for ophthalmic products. It dramatically increases the residual time on the eye from minutes to hours and enables high bioavailability of active pharmaceutical ingredients (API) with excellent tolerability and safety while reducing systemic exposure due to an adequate small drop size.
Limitations of traditional water or oil-based formulations
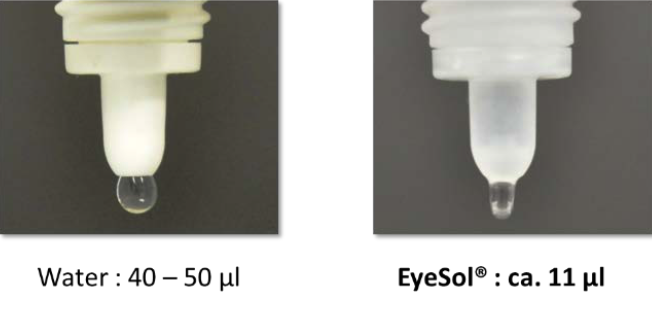
Water-based ophthalmic preparations dispense a typical drop size of 40 to 50 µL. This volume exceeds the external eye’s reservoir capacity, causing spill-over from the eye. The surface tension of water and the outer lipid layer of the tear film further limits its spreading on the ocular surface. In addition, the application of an aqueous-based eye drop can activate the defense mechanism of the eye with rapid blinking after instillation and tear secretion. All these factors reduce the retention time and contribute to a relatively low bioavailability of traditional topical ophthalmic medications, which is reported to be between 1-10%.

Another limitation of using aqueous formulations relates to the fact that up to 60% of today’s new chemical entities are lipophilic compounds or large molecules with poor water solubility. This often requires addition of mineral oils and/or surfactants to the aqueous-formulation that then may cause unwanted side effects like blurred vision. Tolerability can also be affected by preservatives that are often required in multi-dose containers.
Products
Novaliq’s water-free technology opens completely new and intriguing opportunities to cure, relief and prevent diseases in various areas of indications for patients all over the world.
EyeSol® – Proprietary and Unique Water-Free Technology
EyeSol® is based on specific semifluorinated alkanes (SFAs). These compounds have the same refractive index as water and are transparent, inert, non-toxic, amphiphilic liquids that are able to generate novel drug products by utilizing lipophilic and large molecules, such as biologic agents.
-
SFAs have very low surface tension and viscosity and dispense low volume drops (<12 µL) that do not stimulate blinking or reflex tearing. Having both low surface tension and low interface tension. Due to low surface and interface tension, EyeSol® products spread rapidly over the ocular surface and form a flat, transparent layer that enables clear vision without blurring. Because of their amphiphilic nature, EyeSol® also interact with tear film lipids and have been shown to stabilize and restore the tear film.
-
Being aqueous-free, EyeSol® products avoid hydrolytic and oxidative reactions that can degrade active pharmaceutical ingredients, thus improving product stability. In addition, the water-free EyeSol® technology does not support microbial growth, and allows manufacturing of preservative-free formulations in standard multi-dose containers. It also avoids problems of tip clogging that can occur with suspensions.
Novel Drug Products Creation with new composition-of-Matter IP
Our technology is a powerful Intellectual Property (IP) engine
Topical therapies are the therapeutic pillar for many diseases, but traditional water- or oil-based formulations have significant limitations that may influence efficacy and safety.
Safety of EyeSol products
For our products we only use highly pure substances and raw materials from qualified manufacturers. We have extensively tested and proven their safety, efficacy and tolerability in close cooperation with the regulatory authorities. EyeSol products to treat dry eye disease are approved and on the market in the USA, Europe, Australia and New Zealand. Four New Drug Applications for Dry Eye Disease are in regulatory review in China, Europe & Australia. All products are successfully partnered with leading parties worldwide.
Novaliq’s semifluorinated alkanes (SFAs) have been preclinically, clinically and ecotoxicologically tested. SFAs are not metabolized in the human body. Furthermore, they are physically, chemically, and physiologically inert with an excellent biocompatibility and a very good safety profile.
For more information on our products please contact us: info@novaliq.com
Semifluorinated Alkanes (SFAs) are used in healthcare (e.g. in eye surgery) for decades and belong to the group of per- and polyfluoroalkyl substances (PFAS), a family of nearly 5,000 chemicals.
Novaliq uses ultrapure semifluorinated alkanes (SFAs) that are physically, chemically, and physiologically inert with an excellent biocompatibility and a very good safety profile. These substances are not metabolized in the human body. There is further no evidence or indication for any transformation or degradation of SFAs under environmental conditions that may lead to critical substances.
Human safety has further been confirmed in clinical trials and is supported by market data from the use of NovaTears®/ EvoTears™ as medical devices in Europe since Oct 2015, New Zealand since Oct 2017, and Australia since May 2018.
Novaliq offers an industry-leading portfolio
